tudor domain | tudor protein domain tudor domain Tudor domain proteins are molecular adaptors that bind methylated arginine or lysine residues on their substrates. They regulate various aspects of RNA metabolism, chromatin .
Shop these trendy American Optical ORIGINAL PILOT /S Polarized 2-SILVER ST Silver sunglasses online with SmartBuyGlasses Canada. Unbeatable range, fantastic prices .
0 · tudor protein domain
1 · tudor methylligand
2 · tudor methyl ligand recognition
3 · tudor domain definition
4 · tudor dna
5 · tudor arginine methylation
6 · methylarginine
7 · drosophila tudor website
Rum - Amrut Distilleries: The Pioneers Of Indian Single Malt
Learn about the tudor domain, a small protein motif that binds to methylated histones and other molecules. Find chapters and articles on tudor domain functions, interactions, and roles in .
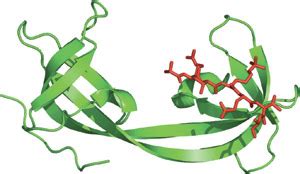
The Tudor domain core contains a conserved β-barrel structure, with an aromatic cage for methyl-ligand recognition. Crystal structures of ligand-bound extended Tudor domains .Tudor domain is a barrel-like structure that recognizes methylated histones and arginines. It is involved in various cellular functions such as DNA methylation, transcription regulation, and .
The Tudor domain comprises a family of motifs that mediate protein-protein interactions required for various DNA-templated biological processes. Emerging evidence demonstrates a versatility .
Tudor domain is a conserved segment of about 60 amino acids that binds to methylated arginine or lysine residues. It is found in proteins that regulate chromatin, RNA, and germline development in eukaryotes. Tudor domain proteins are molecular adaptors that bind methylated arginine or lysine residues on their substrates. They regulate various aspects of RNA metabolism, chromatin . TUD is composed of 11 repeats of the protein motif called the Tudor domain. There are similar proteins to TUD in the germ line of other metazoan species including mice. It encodes a large protein, Tudor (Tud), of 2 515 amino acids, containing 11 copies of a ∼ 60-residue sequence motif, termed the tudor domain. Tudor domains are best .
The Tudor domain is a methyl-lysine and methyl-arginine binding domain present in proteins involved in cellular functions as diverse as DNA transcription, RNA metabolism, gene . The Tudor domain is a conserved protein structural motif of ∼60 amino acids that is characterized by a strongly bent anti-parallel β-sheet composed of five β-strands with a barrel-like fold (Sprangers et al., 2003).The Tudor domain has been reported to recognize and bind methylated lysines and arginines of target substrates (), and this is thought to be the key in .
Tudor domain-containing (TDRD) proteins, the germline enriched protein family, play essential roles in the process of gametogenesis and genome stability through their interaction with the PIWI-interacting RNA (piRNA) pathway. Several studies have suggested the rapid evolution of the piRNA pathway in teleost lineages with striking reproductive diversity. However, there is .
tudor protein domain
tudor methylligand
oculos de sol masculino fendi
Tudor domain proteins function as molecular adaptors, binding methylated arginine or lysine residues on their substrates to promote physical interactions and the assembly of macromolecular complexes. Here, we discuss the emerging roles of Tudor domain proteins during development, most notably in the . We have identified a family of ‘Agenet’ domains that are plant-specific homologs of Tudor domains. This finding has been extended, using a combination of sequence- and structure-dependent approaches, to show that the three β-stranded core regions of Tudor, PWWP, chromatin-binding (Chromo) and MBT domains are homologous because they originate from a .The Tudor domain is a methyl-lysine and methyl-arginine binding domain present in proteins involved in cellular functions as diverse as DNA transcription, RNA metabolism, gene silencing, the transmission of epigenetic posttranslational modifications, and the maintenance of .
Tudor-domain containing proteins have been identified from essentially Tudor domain 1 Current Biology Tudor domain 2 Figure 1. The Tudor domain. Schematic representation of the structure of the two Tudor domains of the human fragile X mental retardation protein FXR2 (PDB: 3H8Z). The structure was generated by the StructuralTudor domain-containing proteins (Tudor proteins), which recognize and bind to methyl-arginine ⁄ lysine residues, play important roles in diverse epigenetics, gene expression and the regulation of various small RNAs. Using the complete set of 23 Tudor proteins from Drosophila, together with the available functional information, we propose a . We focused on identifying binders to the UHRF1 tandem Tudor domain (TTD). Tudor domains are found in 41 human proteins 36 and recognize specific methylated Arg or Lys residues on histones and .
To identify critical Tudor domains required by leukemia, we evaluated the NCBI Conserved Domains Database and summarized 59 Tudor domains in the mammalian genome (span across 36 proteins; data S1) and developed a custom CRISPR library targeting these Tudor domains with 992 sgRNAs (Fig. 1A; ~16.8 sgRNAs per Tudor domain; fig. S1 and data S2).We .Tudor Domains as Methyl-Lysine and Methyl-Arginine Readers. M.V. Botuyan, G. Mer, in Chromatin Signaling and Diseases, 2016 Abstract. The Tudor domain is a methyl-lysine and methyl-arginine binding domain present in proteins involved in cellular functions as diverse as DNA transcription, RNA metabolism, gene silencing, the transmission of epigenetic .
The tudor domain is found in many proteins that colocalise with ribonucleoprotein or single-strand DNA-associated complexes in the nucleus, in the mitochondrial membrane, or at kinetochores. It is not known whether the domain binds directly to RNA and ssDNA, or controls interactions with the nucleoprotein complexes. .
Tudor domain containing protein 3 (TDRD3) is a modular protein identified based on its ability to recognize methylated arginine motifs through its Tudor domain. We have previously shown that TDRD3 . The human Polycomb-like protein PHF1 has been implicated in transcription-regulatory and DNA damage repair pathways. A new study demonstrates that the Tudor domain of PHF1 binds histone H3K36me3 . Dimethylated arginine (DMA) marks are recognized by Tudor domain–containing proteins and play a role in the assembly of ribonucleoprotein complexes. Structural analysis of prototypic Tudor .
In molecular biology, a Tudor domain is a conserved protein structural domain originally identified in the Tudor protein encoded in Drosophila. [1] The Tudor gene was found in a Drosophila screen for maternal factors that regulate embryonic development or fertility. [2]Tudor domains are small 50 amino acid long domains consisting mostly of β-strands [8–11]. The function of tudor domains is not well-established. They are present in a variery of proteins, including the survival motor neuron (SMN) protein, which .A Tudor domain is a protein domain consisting of approximately 60 amino acids forming a barrel-like structure, involved in histone methylation recognition and various cellular functions such as DNA methylation, transcription regulation, and DNA damage repair. What is the Tudor domain? The Tudor domain was first identified as a segment of approximately 60 amino acids that is present in 11 repeated units in the Drosophila protein of the same name. Drosophila tudor was first identified genetically, in a large-scale screen for maternal-effect lethal mutations that affected embryonic development.
The Tudor domain comprises a family of motifs that mediate protein-protein interactions required for various DNA-templated biological processes. Emerging evidence demonstrates a versatility of the Tudor family domains by identifying their specific interactions to a wide variety of histone methylation marks.
The Tudor domain core contains a conserved β-barrel structure, with an aromatic cage for methyl-ligand recognition. Crystal structures of ligand-bound extended Tudor domains (eTuds) have. It encodes a large protein, Tudor (Tud), of 2 515 amino acids, containing 11 copies of a ∼ 60-residue sequence motif, termed the tudor domain. Tudor domains are best characterized by their.
The Tudor domain is a methyl-lysine and methyl-arginine binding domain present in proteins involved in cellular functions as diverse as DNA transcription, RNA metabolism, gene silencing, the transmission of epigenetic posttranslational modifications, and the maintenance of . TUD is composed of 11 repeats of the protein motif called the Tudor domain. There are similar proteins to TUD in the germ line of other metazoan species including mice.
mesa de jantar laca fendi
tudor methyl ligand recognition
Shop Anaconda Malt Liquor black-dynamite baseball t-shirts designed by MindsparkCreative as well as other black-dynamite merchandise at TeePublic.
tudor domain|tudor protein domain