tudor tandem domain of 53bp1 | 53bp1 tandem repair regulator tudor tandem domain of 53bp1 We discovered a protein, Tudor-interacting repair regulator (TIRR), that associates with the 53BP1 Tudor domain and prevents its recruitment to DSBs. Here, we elucidate how . Instead, my quest for Louis Vuitton led me to the age-old muted classic: the Epi style. Epi leather is a Vuitton-specific creation that’s textured with wavy micro-ridges. The only monogram .
0 · tandem tudor domain
1 · 53bp1 tudors
2 · 53bp1 tudor repair regulator
3 · 53bp1 tandem tudor
4 · 53bp1 tandem repair regulator
5 · 53bp1 protein
6 · 53bp1 binding protein
7 · 53bp1 binding partner
LAS VEGAS (KLAS) — The invasion of grasshoppers that took over parts of the Las Vegas valley back in 2019 is still a recent memory for many. 8 News Now spoke with experts to find out if the incident could make a return this year.
Here we show that the major globular domain of the 53BP1 central region adopts a new structural motif composed of two tightly packed Tudor domains and a C-terminal α helix. The newly reported crystal structure of the 53BP1 Tudors in complex with TIRR, together with supporting binding assays using a dually modified (ubiquitinated and . Here we report a 1. 6-Å-resolution crystal structure of the tandem Tudor domain of human 53BP1 bound to a p53K382me2 peptide. In the complex, dimethylated Lys382 is . We discovered a protein, Tudor-interacting repair regulator (TIRR), that associates with the 53BP1 Tudor domain and prevents its recruitment to DSBs. Here, we elucidate how .
Here we report the 1.76-Å crystal structure of TIRR in complex with 53BP1 tandem Tudor domain. We demonstrate that the N-terminal region (residues 10-24) and the L8-loop of . Here we present the electron cryomicroscopy (cryo-EM) structure of a dimerized human 53BP1 fragment bound to a H4K20me2-containing and H2AK15ub-containing .
Here the authors present the crystal structure of Tudor-interacting repair regulator (TIRR) bound to the 53BP1 tandem Tudor domain, which reveals how TIRR blocks H4K20me2 . Here we show that the major globular domain of the 53BP1 central region adopts a new structural motif composed of two tightly packed Tudor domains and a C-terminal α helix.
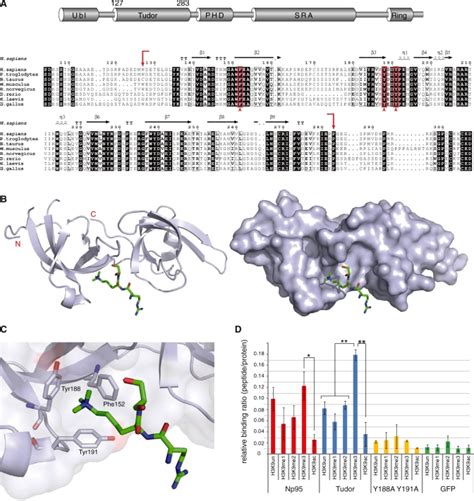
Here, we solved the structure of the domain of 53BP1 that recruits it to sites of DSBs. This domain consists of two tandem tudor folds with a deep pocket at their interface . The 53BP1 tandem Tudor domain is colored in magenta. The N-terminal region and L8-loop of TIRRs (in Tudor-bound or unbound forms), along with the L1, L3, L1′ loops of 53BP1 Tudor are indicated. Since ITC data suggested a 1:1 binding stoichiometry for TIRR and 53BP1 Tudor (Fig. .A tandem tudor domain in human 53BP1 that recognizes methylated residues in the histone core is necessary, but not sufficient, for efficient recruitment.
The cryo-EM density for the tandem Tudor domain of 53BP1 was weaker than the rest of the structure, but its centre of mass was fixed over the tail of H4, . The dimethylated marks p53K370me2 and p53K382me2 are associated with p53 activation or stabilization and both are recognized by the tandem Tudor domain (TTD) of 53BP1, a p53 cofactor. Here we detail the molecular mechanisms for the recognition of p53K370me2 and p53K382me2 by 53BP1. The 53BP1 tandem Tudor domain is colored in magenta. The N-terminal region and L8-loop of TIRRs (in Tudor-bound or unbound forms), along with the L1, L3, L1′ loops of 53BP1 Tudor are indicated .
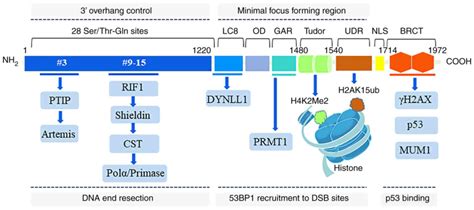
The Solution Structure of the Tandem Tudor Domain (TTD) of 53BP1 in Complex with a p53K370me2 Peptide (A) Schematic representation of the domain architecture of p53 and 53BP1. BRCT, BRCA1 carboxy-terminal repeats; CTD, C-terminal domain; DBD, DNA-binding domain; Olig, oligomerization domain; TA, transactivation domain; TET, tetramerization . Here, we present the crystal structure of the 53BP1 tandem Tudor domain (TTD) in complex with TIRR. Our results show that three loops from TIRR interact with 53BP1 TTD and mask the methylated lysine-binding pocket in TTD. Thus, TIRR competes with histone H4K20 methylation for 53BP1 binding. We map key interaction residues in 53BP1 TTD and TIRR .
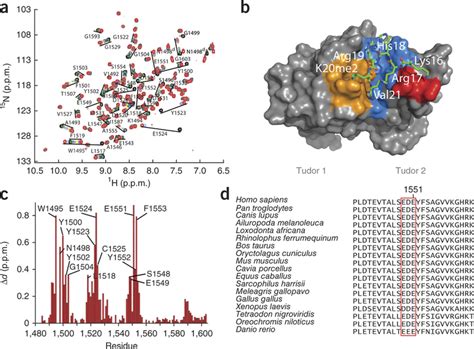
For example, the human ESET protein possesses two Tudor domains connected by a 38 residue linker, while the human GASC1 protein possesses two such domains connected by an 11 residue linker. Alignment of the sequences of these Tudor tandem domains shows that 28 amino acids of 53BP1 TT are conserved in more than 70% of the sequences (Figure 5 .
The 53BP1 tandem Tudor domain (1.0 mM) was incubated with p53-dimethylated lysine peptides [p53K370me2 (residues 366–375), p53K372me2 (residues 367–377), p53K382me2 (residues 377–386)], in a 1:2 molar ratio prior to crystallization. Crystals of the complexes were grown using the microbatch method under oil at 25°C by mixing 2 μl of the . There is an interaction between the tandem Tudor domain of 53BP1 and a histone modification, H4K20me2, found at the break. Here Dipanjan Chowdhury and colleagues identify a new factor, Tudor . Evidence from domain-mapping studies has implicated the interactions between the Tudor domains of 53BP1 and nucleosomes, 53BP1 homo-oligomerization and N-terminal ATM-mediated phosphorylation in .
tandem tudor domain
The methyl-dependent interaction between pRb and the tudor domain containing tumor protein p53 binding protein 1 (53BP1) is described and how this interaction integrates pR b cell cycle control with the DNA damage response is described, widening the repertoire of cellular targets for 53BP1 and suggesting a new role in regulating pRB tumor suppressor activity.
The indicated GST-53BP1 tandem Tudor domain mutants were tested for binding to p53K382me2 as in Fig. 1c. d, an intact tandem Tudor domain is required for robust 53BP1 recognition of p53 in cells. A Western blot analysis as in a in cells expressing the indicated proteins is shown.Tandem tudor domain is present in tumor suppressor p53 binding protein 1 (53BP1) in complex with methylated p53 and H4K20me2 (Roy et al., 2010). SGF29, a part of SAGA subunit, is the only subunit containing a TTD facilitating to recognize H3K4me2/3 (Vermeulen et al., 2010). Here we report the 1.76-Å crystal structure of TIRR in complex with 53BP1 tandem Tudor domain. We demonstrate that the N-terminal region (residues 10–24) and the L8-loop of TIRR interact.
Here we show that the major globular domain of the 53BP1 central region adopts a new structural motif composed of two tightly packed Tudor domains and a C-terminal α helix.
The newly reported crystal structure of the 53BP1 Tudors in complex with TIRR, together with supporting binding assays using a dually modified (ubiquitinated and dimethylated) nucleosome, reveals. Here we report a 1. 6-Å-resolution crystal structure of the tandem Tudor domain of human 53BP1 bound to a p53K382me2 peptide. In the complex, dimethylated Lys382 is restrained by a set of hydrophobic and cation–π interactions in a cage formed by four aromatic residues and an aspartate of 53BP1. We discovered a protein, Tudor-interacting repair regulator (TIRR), that associates with the 53BP1 Tudor domain and prevents its recruitment to DSBs. Here, we elucidate how TIRR affects 53BP1 function beyond its recruitment to DSBs and . Here we report the 1.76-Å crystal structure of TIRR in complex with 53BP1 tandem Tudor domain. We demonstrate that the N-terminal region (residues 10-24) and the L8-loop of TIRR interact with 53BP1 Tudor through three loops (L1, L3, and L1').
Here we present the electron cryomicroscopy (cryo-EM) structure of a dimerized human 53BP1 fragment bound to a H4K20me2-containing and H2AK15ub-containing nucleosome core particle (NCP-ubme) at. Here the authors present the crystal structure of Tudor-interacting repair regulator (TIRR) bound to the 53BP1 tandem Tudor domain, which reveals how TIRR blocks H4K20me2 binding to 53BP1 Tudor and functionally differs from its paralog Nudt16.
Here we show that the major globular domain of the 53BP1 central region adopts a new structural motif composed of two tightly packed Tudor domains and a C-terminal α helix.
53bp1 tudors
Prices start from $65,000ex GST for entry level F300 PRO machines. The fully remote control Green Climber is a commercial mower perfect for carrying out maintenance of green areas, roadsides and highways, particularly in inconvenient or .
tudor tandem domain of 53bp1|53bp1 tandem repair regulator